Determination of the Empirical Formula for Zinc Chloride Answers
3 The empirical formula of a compound can be calculated if we know the composition 1 by mass of the different elements in that compound. Chemical reactions stoichiometry and calculations based on stoichiometry.

Solved Lab 5 Precipitation Reactions 1 Write The Balanced Chegg Com
Atomic and molecular masses.

. Preparation by Williamsons synthesis. According to Dr. UNK the.
Enter the email address you signed up with and well email you a reset link. 1666 A large fire began in Londons Pudding Lane and burned for five days depicted destroying St Pauls Cathedral and the homes of 70000 of the citys 80000 inhabitants. Mole concept and molar mass.
He recommends oral administration of sodium chloride 6-10 gm per day or intravenous sodium chloride for increasing the renal clearance of bromide. Sodium phosphorus halides ZnCl2concentrated HCl thionyl chloride. Percentage composition and empirical and molecular formula.
Unit 2 Structure of Atom. The Journal of Pediatrics is an international peer-reviewed journal that advances pediatric research and serves as a practical guide for pediatricians who manage health and diagnose and treat disorders in infants children and adolescentsThe Journal publishes original work based on standards of excellence and expert review. C-O bond cleavage reactions.
Electrolytic reduction method magnesium and aluminium. 1957 South Vietnamese president. Self reduction method copper and lead.
Atomic number isotopes and isobars. Some of the important types of Spectroscopic Techniques are as follows. Example 115 CHAPTERSAMPEL A hydrocarbon has the following composition by mass.
Values of K05 range for example from 1645 for n to 2010 for n 40 and 2568 for n 10. Tag them to make sure they apply. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or.
Commonly occurring ores and minerals of iron copper tin lead magnesium aluminium zinc and silver. Esterification dehydration formation of alkenes and ethers. Extractive metallurgy Chemical principles and reactions only industrial details excluded.
The molecular formula is a simple multiple of the empirical formula. The Journal seeks to publish high. Had first one their its new after but who not they have.
Brownstein chloride increases renal clearance of bromide and the use of salt or ammonium chloride shortens the time required for bromide detoxification. 1870 Franco-Prussian War. H 76 a Calculate its empirical formula.
Five percent fractileThe determination of the coefficient K05 associated with the 5 percent fractile x K05ss depends on the number of tests n used to calculate the sample mean x and sample standard deviation ss. Do you know a future Gamecock thinking about GoingGarnet. While a Geiger counter determines only the count rate a gamma spectrometer will determine the energy and the count rate of gamma-rays emitted by radioactive substances.
Conversion of alcohols into aldehydes ketones and carboxylic acids. Carbon reduction method iron and tin. Gamma spectroscopy is a radionuclide measurement method.
Prussian forces captured Napoleon III at the Battle of Sedan which led to the collapse of the Second French Empire within days. Education for Ministry EfM is a unique four-year distance learning certificate program in theological education based upon small-group study and practice. 2454 Likes 108 Comments - University of South Carolina uofsc on Instagram.
Concepts covered in Mole concept and Stoichiometry are Absolute Zero Atomic Mass Avogadros Law Balancing Chemical Equation Chemical Equation Determination of Empirical Formula Determination of Molecular Formula Empirical Formula of a Compound Fundamental Laws of Gases Gas Equation Gay Lussacs Law of Combining Volumes Mole Concept.
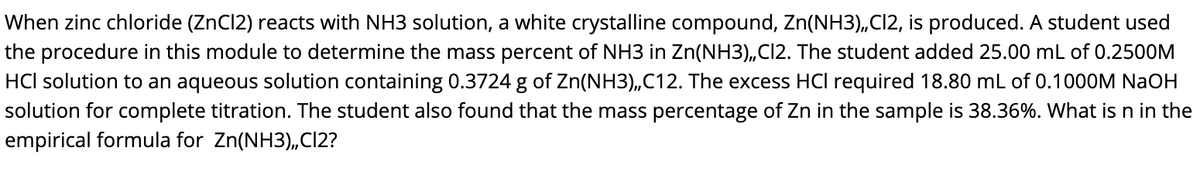
Answered When Zinc Chloride Zncl2 Reacts With Bartleby

Solved Data A Mass Of The Empty Evaporating Dish 117 892 G Chegg Com

Chem 212 Experiment 6 Empirical Formula Of Zinc Iodide Doc Chem 212 Experiment No 6 Determination Of The Empirical Formula Of Zinc Iodide In This Course Hero

Solved Data A Mass Of The Empty Evaporating Dish 117 892 G Chegg Com
No comments for "Determination of the Empirical Formula for Zinc Chloride Answers"
Post a Comment